Bioimprinting of immobilized Lecitase via
Transcrição
Bioimprinting of immobilized Lecitase via
Bioimprinting of immobilized Lecitase via physical coating with polymers in the presence of detergents J.C.S. Santos1,2, N. Rueda1,3, Oveimar Barbosa3, C. Garcia-Galan1, L.R.B. Gonçalves2, R.C. Rodrigues5, and R. Fernandez-Lafuente1* 1Instituto de Catálisis-CSIC, Campus UAM-CSIC, Cantoblanco, Madrid, Spain; 2Departamento de Engenharia Química, Universidade Federal do Ceará, Fortaleza, CE, Brazil; 3Universidad Industrial de Santander, Bucaramanga, Colombia. 4Federal University of Rio Grande do Sul, Porto Alegre, RS, Brazil. 5Instituto de Química Avanzada de Cataluña-CSIC Jordi Girona 18-26, 08034. Barcelona (Spain. *[email protected] Keywords: Enzyme hyperactivation, Detergents, Solid-phase physical modification. INTRODUCTION Lipase activity in aqueous media is reduced due to the opening/closing equilibrium. Detergents may stabilize the open form of lipase, improving its activity. However, they can present some detrimental effects on enzyme stability. Coating of lipases with ionic polymers in the presence of detergents is here proposed as a tool to improve lipase performance. RESULTS AND DISCUSSION negative effect on enzyme stability even at low concentrations (0.01 % (v/v)) and neither SDS can be used for long times at 1 % concentration. In order to keep the hyperactivated form of the enzyme in the absence of detergent (that is deleterious for the stability), ionic polymers were added during the incubation of the immobilized enzyme in different concentrations of detergents. Coating the immobilized enzyme with polyethylenimine (PEI) produced a 3-fold increase in enzyme activity 1 (Figure 1) . However, in the presence of 0.1 % SDS (v/v), this PEI coating produced a 50-fold increase in enzyme activity2. Using an irreversible inhibitor, the inhibition rate showed that the PEI/SDS-CNBrLecitase preparation presented its catalytic Ser more exposed to the reaction medium than the unmodified CNBr-Lecitase. CONCLUSION Figure 1. Effect of SDS concentration and incubation time on the activity of immobilized Lecitase in presence or absence of PEI. Dashed line, empty squares: CNBr-Lecitase incubated with 0.1% SDS, rhombus: CNBr-Lecitase incubated with 0.002% SDS and PEI, circles: CNBr-Lecitase incubated with 0.01% SDS and PEI, triangles: CNBr-Lecitase incubated with 0.05% SDS and PEI, squares: CNBr-Lecitase incubated with 0.1% SDS and PEI. Lecitase Ultra, a phospholipase A1, was covalently immobilized on Cyanogen bromide crosslinked 4 % agarose (CNBr) beads, maintaining 70% of the initial activity. The activity of the immobilized enzyme, Lecitase-CNBr, in the presence of detergents, such as Triton X-100, sodium dodecyl sulphate (SDS), cetyltrimethyl ammonium bromide (CTAB) increased, up to 800 % when using CTAB. However, CTAB and Triton-X100 presented a The coating of immobilized Lecitase Ultra with PEI in the presence of SDS permits the stabilization of the open form of the enzyme, increasing th activity of the immobilized enzyme even by a 50 fold factor. ACKNOWLEDGEMENTS We gratefully the Spanish Government, grant CTQ2009-07568 and CTQ2013-41507-R and CNPq (Brazil) for financial support. The authors wish to thank Ramiro Martínez (Novozymes, Spain) for kindly supplying the enzymes used in this research. REFERENCES [1] Santos, JCS; Garcia-Galan, C; Rodrigues, RC; Ana HBS; Goncalves,LRB; Fernandez-Lafuente, R. Enzyme Microb Technol 2014;60:1–8. [2] Santos, JCS; Garcia-Galan, C; Rodrigues, RC; Ana HBS; Goncalves,LRB; Fernandez-Lafuente, R. Process Biochem DOI: 10.1016/j.procbio.2014.05.009. VII Workshop on Biocatalysis and Biotransformations and 1o Simposio Latinoamericano de Biocatalisis y Biotransformaciones




















 Enzymes in non-aqueous solvents.
Enzymes in non-aqueous solvents.
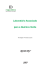 Enzymes in non-aqueous solvents.
Enzymes in non-aqueous solvents.