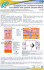quality evaluation of finasteride manipulated capsules
Transcrição
quality evaluation of finasteride manipulated capsules
QUALITY EVALUATION OF FINASTERIDE MANIPULATED CAPSULES: THE IMPORTANCE OF EXCIPIENTS STANDARDIZATION SANTOS, O.M.M1, SANTOS, A.L.A.,1 BONFILIO, R.,2 ARAÚJO, M.B. de1, 1 Faculdade de Ciências Farmacêuticas da Universidade Federal de Alfenas, Alfenas-MG, Brazil. Departamento de Fármacos e Medicamentos, Universidade Estadual Paulista, Araraquara, SP, Brazil. Email: [email protected]. 2 Dissolution profile, finasteride, capsules, physico-chemical analysis. 1. Introduction The quality control analyses performed on products manipulated in pharmacies is extremely important to ensure physical and chemical quality of these products, which will guarantee efficiency, safety and credibility. Finasteride (FNS) is a drug used for the treatment of benign prostatic hyperplasia and androgenetic alopecia and it is widely prescribed in Brazil in capsules form. The objective of this work is to evaluate the physico-chemical quality of finasteride capsules in order to check the need for standardization of excipients used in the manipulation process. 2. Methods The pharmaceuticals used were Proscar® 5 mg tablets and three different products in capsules, which were coded as product A, product B and product C. The samples were analyzed by tests of average weight, disintegration time, finasteride content, content uniformity test and dissolution test. 3. Results Only the product C showed dissolution profile similar to the reference product in 30 minutes (Figure 1). Table 1. Physico-chemical test results of tested products Test Results a Proscar Product Product Product 5mg A B C Average Aproved Aproved Aproved Aproved weight (mg) Disintegra3 3 2 2 tion time minutes minutes minutes minutes Finasteride 98.8 95.8 95.9 97.7 content (%) Content Aproved Aproved Aproved Aproved uniformity (%) Dissolution Aproved Reproved Reproved Aproved test (%) a U.S.P1 requirements: average weight (mg): ± 10.0%; disintegration time: 45 min; finasteride content: 95.0 to 105.0%; content uniformity: 85.0 to 115.0%; dissolution test: each unit ≥ 85.0%. Table 2. Excipients used in tested products Proscar® Product Product A B Lactose, starch, Lauryl Starch yellow ferric sodium and talc oxide, docusate sulphate sodium, cellulose, (0.5%), talc, Mg stearate, lactose, Cellulose methyl talc, hydroxypropyl, starch titanium dioxide. and aerosil Product C Lauryl sodium sulphate (1.5%), Mg stearate, aerosil, talc and starch 4. Conclusion Although the products A and B presented appropriate drug contents, these products failed the dissolution test. These results indicated that the excipients interfering with the in vitro release of finasteride, which highlights a need for excipients standardization. Acknowledgments To N.C.Q./UNIFAL-MG. Figure 1. Dissolution profiles of Proscar® 5 mg, product A, product B and product C. 1 UNITED STATES PHARMACOPEIA, 32. ed. The United States Pharmacopeial Convention, Rockville, MD, 2009.
Documentos relacionados
Book of Abstracts - Rodomiro Ortiz and Nagib Nassar
 M-04: D.D. Ngudi Konzo and cassava toxicity – a study of associated nutritional factors in the
Popobaka district of the Democratic Republic of Congo
M-04: D.D. Ngudi Konzo and cassava toxicity – a study of associated nutritional factors in the
Popobaka district of the Democratic Republic of Congo